Process Development
What is needed at a minimum to establish credibility in the scientific community is compelling thermodynamic and kinetic data for any proposed system.
Before any research work can begin, it is obvious that a comprehensive literature review must be done. Any pertinent thermodynamic data must be critically evaluated. The feed material must be realistically characterized in terms of physical and chemical properties. The stoichiometry and phase relations for the system must be known.
The thermodynamic properties of the system should be determined experimentally. The extent of deviation from calculated thermodynamic values for condensed and vapor phases should be measured. Appropriate phase diagrams should be constructed relating phase composition, free energy of formation, and temperature; e.g., phase diagrams for the elements in the ilmenite (Fe- Ti-O) and for the products of the ilmenite reduction process (Fe- Ti-O2-H2) for temperatures between 700 and 1000°C. The vapor phase and residue should be accurately analyzed as well.
If the thermodynamic data indicate an attractive extraction proGess, the kinetics and heat and mass transfer properties must next be systematically investigated.
It should be noted that experimental programs for high- temperature processes are extremely difficult and may require several years' effort.
Assuming that the proposed process has been demonstrated by such a bench-scale program to be feasible, it is appropriate before proceeding further to do an economic evaluation of the process. Operating and capital costs should be assessed, and special considerations, such as mass and energy requirements, need to be carefully analyzed.
If the process still appears attractive, then a terrestrial pilot plant is mandatory. If the process does not work on Earth, it probably cannot be made to work on the Moon. A great deal of consideration would have to go into designing a pilot plant that would yield useful information regarding a plant at a nonterrestrial site. Even for the very simplest of processes, it is clear that its development for use on the Moon would require an intensive research and development program. If begun now, the most optimistic program for an ideal process would probably take 20-50 years and involve hundreds of millions of dollars.
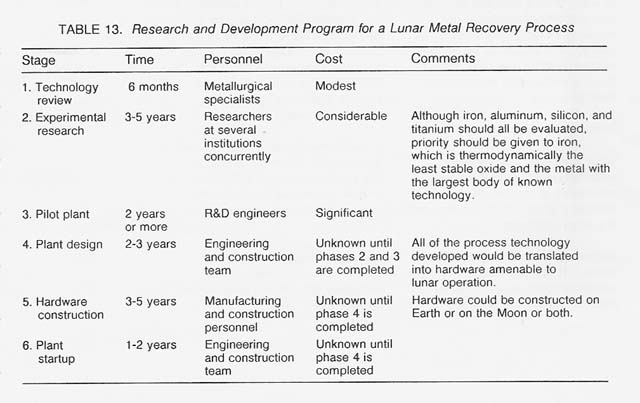
Thus, if lunar resources are to be used in the early 21 st century, there is a clear need to begin a research and development program now. It should proceed through the stages presented in table 13.

A fair amount of speculation on metal recovery processes has been made, but so far little of the work can stand up to scientific scrutiny. To build a workable lunar materials recovery plant, the logical program described must be followed. Sufficient amounts of capital and work time by qualified metallurgical personnel must be provided.
Experts in the fields of mineral science and metallurgy must contribute to the development of the required new process technology. The most effective means of achieving this objective is by joint cooperative efforts between established metallurgical experts and specialists in the planetary sciences. If the space program attempts to develop metallurgical capabilities in-house, the timing objectives for the new technology will be impossible to meet.
Applicability of Space Technology to Terrestrial Metal Processing
Metallurgical technology on Earth has developed slowly with a limited base of trained technologists and R&D funding. The research is very difficult experimentally, complex, and hard to scale up. Hence, process development is slow.
It seems obvious that even a minor R&D effort by the space program would be a major contribution to the existing bodyof knowledge. Although it is impossible at this time to judge what metal processing technologies will be developed for space in the next 25 years, it is of interest to use some general guidelines to speculate, as follows.
Chloride and fluoride systems offer interesting potential for metal extraction. Such processing schemes would provide a technology base for a terrestrial metallurgical breakthrough that would reduce energy consumption per unit output. Along with the process chemistry, the development of recycling technology could clearly be advantageous in dealing on Earth with ever increasing environmental limitations.
Development of electrochemical, electrode, or electrolysis technologies for metal extraction, refining, or processing could advance the currently rather empirical state of the art on Earth for certain metals.
The development of a computer interface with metallurgical process technology would likely advance the state of metallurgy by several orders of magnitude and is one of the most exciting prospects for change.
Metals produced by new technology on the Moon will not contain the solutes usually contained in their counterparts on Earth, such as carbon and sulfur in steel. Physical and mechanical properties of a whole new cadre of alloys will be measured. The creation of such uncontaminated alloys will advance the field of materials science since the effect of impurities on properties can be zeroed out. Such new alloys may be used on Earth for unique applications, now impossible to fulfill.
And new metallurgical technology developed to extract metals from alternate mineral feedstocks could have strategic and possibly commercial value on Earth.
References
Dresher, W. H. 1974. Metallurgical Engineering in the United States: A Status Report. J. Metals 26 (5): 31.
Rao, D. Bhogeswara; U. V. Choudary; T. E. Erstfeld; R. J. Williams; and Y. A. Chang. 1979. Extraction Processes for the Production of Aluminum, Titanium, Iron, Magnesium, and Oxygen from Nonterrestrial Sources. In Space Resources and Space Settlements, ed. John Billingham, William Gilbreath, and Brian O'Leary, 257-274. NASA SP-428.
Richardson, F. D., and J. H.. E. Jeffes. 1948. Thermodynamics of Substances of Interest in Iron and Steelmaking from 0 to 2400 Degrees. J. Iron & Steellnst. (London) 160:261-270.
Skinner, Brian J, 1976. A Second Iron Age Ahead? American Scientist 64:258.
Wanke, H., et al. 1970. Major and Trace Elements in Lunar Material. Proc. Apollo 11 Lunar Sci. Cont., 1719-1727. Pergamon Press.